Action potentials are the principal mechanism of nerve impulse propagation and transmission, and they allow depolarization at a single region of skeletal and cardiac muscle cells to spread across the entire cell. Action potentials require a stimulus that depolarizes the cell membrane potential to a threshold value, and then a sufficient density of voltage-gated channels to generate an electrical response. An action potential results from a sequential change in ion permeabilities. An action potential generated by a nerve axon is used to illustrate the characteristic changes in ion permeabilities and membrane potential.
The fast Na+ channel is a voltage-gated structure with two gates that can block the lumen of the channel. The transition voltage for gate movement is around -70 mV, the threshold voltage. Both gates move whenever the membrane potential depolarizes or hyperpolarizes through this transition voltage value, but the gates move at different rates. The activation m-gate moves immediately, and the inactivation h-gate takes longer to get into position. At a resting membrane potential of -90 mV, the m-gate blocks the channel and the h-gate is open. As the membrane potential depolarizes past threshold, the gates move, with the m-gate opening the channel and the h-gate starting to close the channel. The channel remains open for a period of time until the h-gate is in the closed position. During repolarization through -70 mV, the gates again shift. The m-gate moves to block the channel, and the h-gate is still in place blocking the channel. Both gates block the channel until the h-gate moves to the open position. This gate dynamic results in a period of time during depolarization to threshold when both gates are open and Na+ permeability is increased.
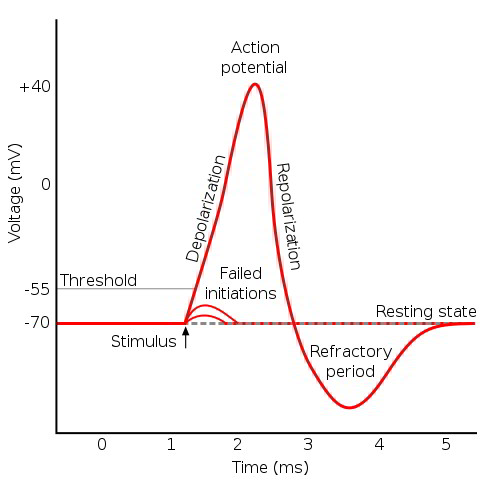
The resting membrane potential results from the high membrane permeability to K+ relative to Cl–, Na+, and Ca++. A stimulus generates a local potential that depolarizes a region of the membrane past the threshold value. The voltage-sensitive m-gate of the fast Na+ channel opens, depolarizing the membrane. This depolarization opens more voltage-gated fast Na+ channels, further increasing Na+ permeability and further depolarizing the membrane in a positive feedback cycle. An action potential is generated if the density of functional fast Na+ channels is sufficient to allow the progressive depolarization.
The Na+ channels remain open for a limited period of time and then close as the h-gate inactivates the channel. Movement of the h-gate is also voltage dependent and is initiated by the membrane depolarizing past the threshold. One major difference is in the speed of movement. The h-gate closure is a slower event.
Even slower are the voltage-gated K+ channels, which do not fully reach their open position until after the peak of the action potential. As the K+ channels finally open, the membrane potential shifts toward the K+ Nernst equilibrium value, possibly hyperpolarizing the membrane potential compared with the original resting membrane potential. The membrane potential returns toward the resting membrane potential as the voltage-gated K+ channels close. As the membrane potential drops below -70 mV, the fast Na+ channel gates reset. The m-gate quickly moves to block the channel, and the h-gate more slowly unblocks the channel. In contrast to the depolarizing event, the fast Na+ channel is blocked by one or both gates during the repolarization phase.
The movement of the activation and inactivation gates of the voltage-gated Na+ channel sets limits on the responsive-
ness of the cell. The cell will not generate an action potential until after the fast Na+ channel m-gate is reset. The period of time when the cell is unable to generate an action potential is called the absolute refractory period. A relative refractory period follows the absolute refractory period, and it persists until the voltage-gated K+ channels close. During this time, the cell requires a greater than normal stimulus to reach threshold and generate an action potential. Action potentials of different tissues have characteristic forms and durations, based on the presence and density of fast Na+ channels and the timing of the K+ channel opening.
Threshold requires a critical density of open voltage-gated Na+ channels so that the electrical depolarization becomes self-sustaining. A gradual depolarization allows some channels to open and inactivate without reaching critical current flow. Consequently, rapid depolarization more effectively generates an action potential than a more gradual depolarization does.
Changes in intracellular or extracellular ion concentrations will modify the action potential. The action potential is the result of changing ion permeabilities, driving the membrane potential toward that ion’s Nernst potential. Increases in intracellular Na+ or decreases in extracellular Na+ will decrease the Na+ Nernst potential and diminish the overshoot of the action potential. Decreases in intracellular K+ or increases in extracellular K+ will decrease the K+ Nernst potential, and because of the high resting K+ permeability, these changes will depolarize the resting membrane potential. Cl– distributes passively based on the resting membrane potential, and changes in extracellular Cl– will not greatly affect the action potential.
Maintained changes in ion permeabilities also will affect the resting membrane potential and the action potential. Maintained increases in Na+ permeability will depolarize the cell, bringing the membrane potential closer to the threshold. Maintained increases in K+ permeability will hyperpolarize the cell and move the resting membrane potential farther from the threshold. Maintained increases in Cl– permeability have little effect on the resting membrane potential but will diminish the changes in membrane potential caused by alterations in the permeabilities of the other ions. This includes diminishing the Na+-mediated overshoot and diminishing the K+-mediated hyperpolarization.

 (48 votes, average: 4.79 out of 5)
(48 votes, average: 4.79 out of 5)