Passive transport describes the mechanism of transport of substances along the gradient without expense of any energy It relies on the physical aspects like concentration gradient, electrical gradient and pressure gradient. Given that the transport of substances takes place along the gradient, this process is likewise called down-hill movement The passive transport mechanisms operating at the cell membrane level are diffusion and osmosis.
DIFFUSION
Diffusion describes passive transport of molecules from locations of greater concentration to locations of lower concentration. Diffusion through cell membrane is divided into 2 subtypes called: easy diffusion and facilitated diffusion.
Simple Diffusion
In easy diffusion, transport of atoms or molecules takes place from one place to another due to their random movement Due to consistent random movement, the molecules hit each other as well as strike with the cell membrane. The frequency of accident and the possibility of striking to the cell membrane will be greater on the side of the membrane having greater concentration of that specific molecule In this process there takes place a net flux of the molecules from the locations of high concentration to locations of low concentration.
The net movement of the molecules stops when the concentration of molecules equates to, and there takes place a condition of diffusional balance. Quantitatively, the net movement of the molecules across a permeable membrane where just easy diffusion is happening is revealed by Fick’s law of diffusion, which mentions that rate of diffusion (J) is straight proportional to the distinction in the concentration of the substance in 2 areas (concentration gradient, i.e. C1 − C2) and cross-sectional area (A) and inversely proportional to the range to be taken a trip, i.e. thickness of the membrane (T).
Therefore, 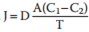
The diffusion of molecules across the biological membranes varies relying on the lipid solubility, water solubility, type of electrical charge and size of the molecules Even more, selective permeability of the semipermeable cell membrane likewise impacts the diffusion of various molecules How the various molecules diffuse across a cell membrane are gone over listed below.
Simple Diffusion of Lipid Soluble Substances across The Cell Membrane
The rate of diffusion through the lipid bilayer of the cell membrane is straight proportional to the solubility of a substance in lipids. For that reason, molecules of substances like oxygen, nitrogen, co2, alcohol, steroid hormones and weak natural acids and bases, being lipid soluble, scattered really quickly through the lipid bilayer of the cell membrane.
Simple Diffusion of Water and Other Lipid Insoluble Molecules Through The Cell Membrane
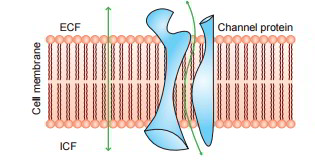
Amazingly, water and other lipid insoluble substances can likewise pass quickly through the cell membrane. It has actually been revealed that it is possible due to the existence of the so-called protein channels (made from transmembrane proteins) in the cell membrane.
Diffusion Through Protein Channels
The protein channels are tube-shaped channels which extend in the cell membrane from the extracellular to the intracellular ends. For that reason, even the extremely lipid insoluble substances can diffuse by easy diffusion straight through these channels of the cell membrane. The protein channels have actually been geared up with following characteristics: Selective permeability and Gating mechanism.
Selective Permeability of Protein Channels
The protein channels are extremely selective, i.e. each channel can allow just one type of ion to go through it. This arises from the characteristics of the channel itself, such as its size, its shape and nature of electrical charges along its within surfaces Examples of some selective channels are: Sodium channels are particularly selective for the passage of sodium ions. These are 0.3 by 0.5 nm in size and their inner surfaces are highly adversely charged. Potassium channels are particularly selective for the passage of potassium ions. These are 0.3 by 0.3 nm in size and are not adversely charged.
Gating Mechanism In Protein Channels
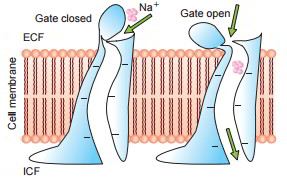
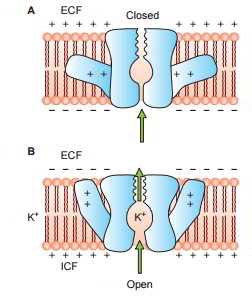
Some protein channels are constantly open, whereas a lot of others are ‘gated’, i.e. they are geared up with real gate-like extensions of the transport protein molecule which can open and close according to requirement. This gating mechanism is a way of managing the permeability of the channels The opening and closing of gates are managed by 3 primary methods:
- Voltage-gated channels.
These react to the electrical potential across the cell membrane. As displayed in the figure When it comes to sodium channels evictions lie at the external end of the channels and these stay firmly closed, when there is a strong negative charge on the within cell membrane. When the within cell membrane loses its negative charge, these gates open and there takes place a significant inflow of sodium ions. This is the basis of incident of action capacities in nerves that are accountable for nerve signals.
When it comes to potassium channels, evictions lie at inner end of the channel and they too open when within the cell membrane loses its negative charge, however, this response is much slower than that for sodium channel. The opening of potassium channel gates is instrumental for ending the action potential.
- Ligand-gated channels Gates of these channels open when some other chemical molecule binds with eviction proteins that is why this is likewise called chemical gating. Among the most essential example of ligand channel gating is the impact of acetylcholine on the so-called acetylcholine channels This gate plays an essential role in transmission of nerve signals from one afferent neuron to another and from nerve cells to muscle cells.
- Mechanical-gated channels Some protein channels are opened by mechanical stretch. These mechano-sensitive channels play an essential role in cell movements.
Facilitated Diffusion
The water soluble substances having bigger molecules such as glucose can not diffuse through the protein channels by easy diffusion. Such substances diffuse through the cell membrane with the aid of some carrier proteins For that reason, this type of diffusion is called facilitated or the carrier moderated diffusion. There are lots of types of carrier proteins in the cell membrane, each type having binding sites that specify for a specific substance Amongst the most essential substances that cross cell membranes by facilitated diffusion are glucose and the majority of the amino acids.
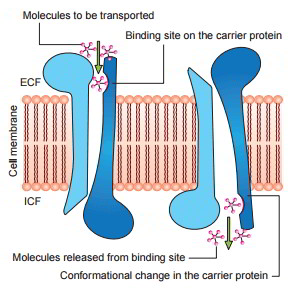
Mechanism of Facilitated Diffusion
Postulated mechanism for facilitated diffusion is displayed in figure As displayed in the figure, a conformational modification takes place in the carrier protein after the molecule to be transferred is bound at the receptor site The repeated spontaneous configurationally modifications enable the diffusion of the molecule.
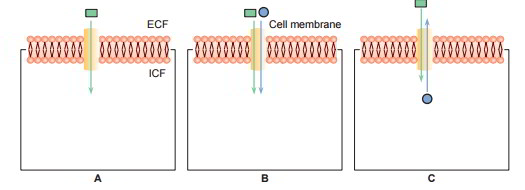
Types of Carrier Protein Systems
3 types of carrier protein systems are understood: uniport, symport and antiport. The symports and antiport are together referred to as co- transport.
- Uniport: In this system the carrier proteins transport just one type of molecules (A).
- Symport: In this system transport of one substance is related to transfer of another substance For instance, facilitated diffusion of glucose in the renal tubular cells is related to the transport of sodium (B).
- Antiport: In this system the carrier proteins exchange one substance for another. For instance, Na+– K+ exchange or Na+– H+ exchange in the renal tubules (C)
Difference Between Simple And Facilitated Diffusion
- Uniqueness. The carrier proteins are extremely particular for various molecules.
- Saturation. In easy diffusion the rate of diffusion increases proportionately with the boost in the concentration of the substance and there is no limitation to it. Nevertheless, in facilitated diffusion the rate of diffusion increases with boost in concentration gradient to reach a limitation beyond which a more boost in the diffusion can not happen. This is called saturation point and here all the binding sites on the carrier proteins are inhabited and the system runs at its optimum capability.
- Competitors. When 2 molecules, state A and B are brought by the exact same protein there happen a competitors in between the 2 molecules for thetransport Therefore, a boost in the concentration of ‘A’ molecule will reduce the transport of molecule ‘B’ and vice versa. No such competitors is understood to happen in easy diffusion
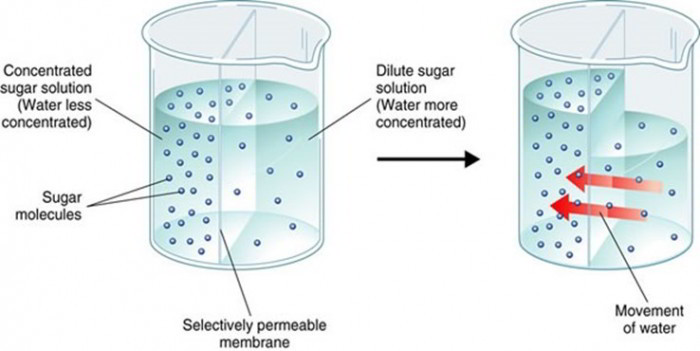
Osmosis
Osmosis describes diffusion of water or other solvent molecules through a semipermeable membrane (i.e. membrane permeable to solvent however not to the solute) from a solution including lower concentration of solutes to the solution including greater concentration of solutes; Figure listed below shows osmosis across a selective permeable membrane. When a sodium chloride solution is put on one side of the membrane and water on the other side the net movement of water takes place from the pure water into the sodium chloride solution.
Osmotic Pressure
Osmotic pressure describes the minimum pressure which when used on the side of greater solute concentration avoids the osmosis. When suitable pressure is used the net diffusion of water into the sodium chloride solution is avoided. The osmotic pressure in the body fluids describes the pressure applied by the solutes dissolved in water or other solvents.
The osmotic pressure applied by the colloidal substances in the body is called colloidal osmotic pressure The colloidal osmotic pressure due to plasma colloids (proteins) is called oncotic pressure The osmotic pressure relies on the variety of molecules or ions dissolved in a solution instead of their size, type or chemical composition.
Osmole, Osmolality and Osmolarity
Osmoleis the unit utilised in place of grams to reveal the concentration in regards to variety of osmotically active particles in an offered solution One osmole amounts to the molecular weight of a substance in grams divided by the variety of easily moving particles freed in solution by each molecule Therefore:
- A molar solution of glucose consists of 1 mole and puts in osmotic pressure of 1 atm.
- A molar solution of NaCl consists of 2 osmoles (1 mole of Na+ and 1 mole of Cl −) and puts in osmotic pressure of 2 atmospheres.
- A molar solution of CaCl2 consists of 3 osmoles (1 mole of Ca2+and 2 moles of Cl −) and therefore puts in osmotic pressure of 3 atm.
- One milli osmole (mOsm) is 1/1000of an osmole.
Osmolality of a solution describes the variety of osmotically active particles (osmoles) per kg (kg) of a solution, whereas, osmolarity describes the variety of osmoles per litre (L) of asolution For that reason, osmolarity is impacted by the volume of the different solutes in the solution and the temperature, while the osmolality is not.
The osmotic pressure is identified by the osmolality and not the osmolarity. Nevertheless, the quantitative distinctions in between the osmolarity and osmolality are less than 1%. In practice, osmolarity is more often utilised in physiological research studies, considering that it is much more simpler to determine osmolarity vis-a-vis osmolality.
Normal plasma osmolality: The typical osmolality of the extracellular and intracellular fluids is 290 milliosmoles per kg (mOsm/kg). In the plasma of the overall osmolality 270 mOsm are contributed by Na+, Cl − and HCO3−. The staying 20 mOsm are contributed by glucose and urea. Since of the big molecular weight and thus lower variety of particles, plasma proteins (70g/L) contribute 2 mOsm to the overall plasma osmolality.
Tonicity of Fluids
In clinical practice, the word tonicity constantly describes tonicity of a solution with regard to that of plasma (290mOsm). Simply puts, it is the red blood cell (RBC) membrane across which the tonicity is checked.
Therefore:
- Isotonic fluids are those which have osmolality just like plasma. RBCs neither diminish nor swell in such solution A solution of 0.9% NaCl is isotonic with plasma.
- Hypertonic fluids have osmolality greater than the plasma. The RBCs diminish in such options by losing water by osmosis.
- Hypotonic fluids are those whose osmolality is lower than that of plasma. The RBCs inflate in hypotonic options by getting water by osmosis.

 (54 votes, average: 4.88 out of 5)
(54 votes, average: 4.88 out of 5)